I still have some clarifying questions for him but he's already made his case!
For what it's worth, here is the Cornell Pour Thru method:
Steps for the PourThru method:
1. water containers to saturation (so that a few drops of water come out of the bottom of the container) with the normal irrigation water they have been receiving
2. after container has drained for one hour, place a saucer under the container
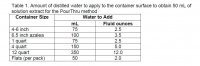
3. pour enough distilled (DI) water on the surface of the container to get 50 mL (1.5 fluid ounces) of leachate to come out of the bottom of the container (Table 1)
4. collect leachate for pH and EC testing
5. calibrate pH and EC meters
6. measure pH and EC of samples
See table 1
INTERPRETING TEST DATA
EC (Electrical Conductivity)
The values that you measure for EC will depend on the method you use for testing the container media. EC
guidelines for several horticulture crops are presented in the table on page 8.
Problems with Low EC
A low EC means that your plants are not getting enough fertilizer salts. Symptoms can include stunted plant growth or leaf discoloration due to lack of nutrients. Nitrogen deficiency (yellowing of lower leaves) often appears first.
Problems with High EC
Excess salts can accumulate when: you are applying more fertilizer than the plant requires; the container media has a high initial salt level; leaching during irrigation is insufficient; or your water source contains naturally high levels of salts (bicarbonates,calcium, chloride, magnesium, sodium, or sulfates). Excess salts can cause tissue death. Symptoms often appear first on the lower leaves and appear as yellowing (chlorosis) or browning (necrosis) that begins at the edges of the leaves and spreads inward. High salts can cause root tips to die back; and plants may show wilting even though the medium is still moist. High salt levels have been shown to increase the incidence of Pythium root rot.
pH
pH affects the ability of nutrients to dissolve in water (solubility). Solubility is important because roots can only take up nutrients that are dissolved in solution and cannot take up the solid form of the nutrient. The graphs on page 5 show nutrient solubility in container media (left) and of soil (right) in relationship to pH.
Problems with Low pH
In container media, the micronutrients iron, manganese, zinc, and boron are highly soluble at low pH (pH 5.0-6.0). Therefore, at low pH these nutrients are available and readily taken up by roots. If pH is too low, typically below 5.0 for most plants, the nutrients become so soluble that they may be taken up at harmful or toxic concentrations. A classic symptom of this is iron toxicity which appears as leaf bronzing and chlorosis which appear first on lower leaves. Certain plants that are especially efficient at taking up iron, such as seed and zonal geraniums and marigolds, can exhibit iron toxicity when pH is below 6.0.
Problems with High pH
At high media pH the low solubility of phosphorus, iron, manganese, zinc, and boron makes these nutrients less available to be taken up by roots and so deficiency symptoms can occur. Certain plants are less efficient at absorbing micronutrients (especially iron and manganese). These plants require a slightly lower pH to be able to absorb enough of these nutrients. A classic example of this is iron deficiency is petunia. Affected plants show yellowing between the veins on the upper leaves. Often there is enough iron provided in the fertilizer/container media, but the pH is too high for roots to absorb it.
LONG-TERM MONITORING
Sampling container media for pH and EC is most effective when samples are taken periodically during crop production as opposed to measuring at only 1 time point. This allows you to look for trends. If pH or EC begin to creep outside of the preferred range, then action can be taken to bring these under control. In the example below, bedding plants were grown with a complete fertilizer mix (21-5-20 N:K2O 2O5) at 3 different levels of nitrogen.



 I bought another big bad of coco when I was at the store buying my pH meter so I am committed for a while.
I bought another big bad of coco when I was at the store buying my pH meter so I am committed for a while.