Hey I hear that. pH the power of the Hydrogen Ion. I am a newbie at growing. and i was using plain ole tap water and not checking it. when i lowered the ph to around 6.5 and fed my 1st grow. jeez when i checked on them the next day. it was if they were saluting me. standing all erect and showing off lol. nice post McBudzwell said.
Navigation
Install the app
How to install the app on iOS
How To Use Progressive Web App aka PWA On 420 Magazine Forum
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
What is pH?
- Thread starter Racefan
- Start date
Doctor Trevor
Well-Known Member
I actually adjust the pH of my plants' water and nute solution down to 5.2 to 5.3.
YMMV.
YMMV.
Old Guy Teaches
Well-Known Member
I go to 6.1 here... have had a meter a Long time. about a 100 Bucks and worth every penny. My water here in Bev Hills comes out at 7.4 ish. and changes drastically...
So, I'm not the author of this, but I found it highly informative and thought I would share it. I'm not a chemist, my understanding is somewhat limited on it, so I won't be able to answer much in the way of questions that's not already in the below text.
Understanding PH & Hydroponics | by Manic Botanix
PH stands for the power of hydrogen, although many refer to the meaning of pH as ‘potential hydrogen’. Either way, potential hydrogen or power of hydrogen, pH is a parameter that measures the acidity or alkalinity of a solution by measuring the hydrogen ion concentration in solution. A pH value indicates the relationship between the concentration of free ions H+ (hydrogen) and OH- (hydroxide) present in a solution. Put simply, if a solution is very acidic, there will be lots of active hydrogen ions and hardly any hydroxide ions. If a solution is very alkaline, the opposite is true. In pure water, the concentrations of hydrogen and hydroxide ions are about the same. Therefore, pure water has a pH that is neutral at pH 7.0.
The pH scale is a logarithmic scale that typically runs from 1 to 14. Each whole pH value below 7 (the pH of pure water) is ten times more acidic than the higher value and each whole pH value above 7 is ten times less acidic than the one below it. For example, a pH of 5 is ten times more acidic than a pH of 6 and 100 times (10 times 10) more acidic than a pH value of 7. So, a strong acid may have a pH of 1-2, while a strong base may have a pH of 13-14. See following illustration of the pH scale.
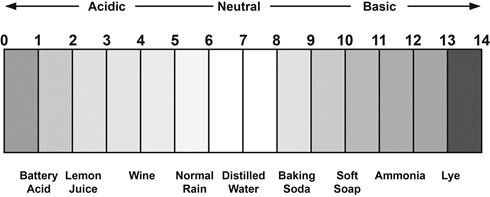
Why does pH change in nutrient solutions?
This comes back to understanding the power of hydrogen, otherwise known as potential hydrogen in solution and some of our earlier material on EC where we touched on positively charged cations and negatively charged anions in hydroponic solutions. Other than this, pH changes largely occur due to the principle of electroneutrality where chemical reactions take place on an equivalent basis. The law of electroneutrality states that in any single ionic solution (e.g. a hydroponic nutrient solution) a sum of negative electrical charges attracts an equal sum of positive electrical charges. Therefore, according to the principle of electroneutrality, the total charge of an aqueous solution must be zero. For this to occur, the number of positive charges contributed by cations must be equal to the number of negative charges contributed by anions.
Based on this, in very simple terms, when a plant removes a positively charged cation from the nutrient reservoir/tank it leaves a negatively charged anion in its place and when a plant removes an anion from the nutrient reservoir/tank it leaves a cation in its place. See following images.
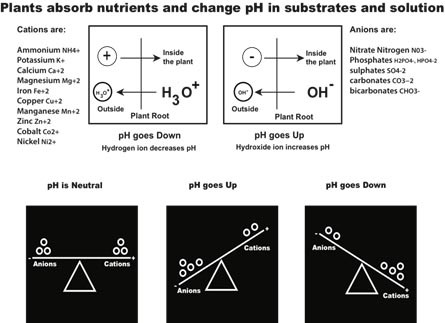
Since every macro and micro element ion in solution has an electrical charge plants can’t just take them, otherwise the electrical equilibrium would be out of balance. What plants do is swap them with equivalent amounts of H+ and OH- ions.
For example:
Ammonium N (NH4+) is swapped with 1xH+
Nitrate N (NO3-) is swapped with 1xOH-
Potassium (K+) is swapped with 1xH+
Calcium (Ca++) is swapped with 2xH+
Magnesium (Mg++) is swapped with 2xH+
Iron (Fe++) is swapped with 2xH+
Manganese (Mn++) is swapped with 2xH+
Zinc (Zn++) is swapped with 2xH+
phosphates (HPO4–) is swapped with 2xOH-
In this way electrical charge equilibrium remains the same.
The ratio in uptake of anions and cations by plants may cause substantial shifts in pH. In general, an excess of cation over anion leads to a decrease in pH, whereas an excess of anion over cation uptake leads to an increase in pH. That is, when the anions are uptaken in higher concentrations than cations the plant excretes OH- or HCO3- anions to balance the electrical charges inside, which increases the pH value. For example, if a plant absorbs the negatively charged nitrate nitrogen (NO3-) heavily it will start to contribute more OH – than H3O + ions into the solution and the result will be an increase in pH. On the other hand, if the plant absorbs high levels of the positively charged potassium (K+) it will contribute more H3O + than OH – ions and the result will be a decrease in pH.
This phenomenom is frequently seen where plants are grown with a full spectrum nutrient solution that contains nitrogen either as ammonium nitrogen (NH4+) or nitrate (NO3– ) nitrogen. When plants are fed only with NH4+, cation uptake generally exceeds anion uptake and the pH of the substrate decreases. On the other hand, when the plant is fed only with NO3– the uptake of anion to cation ratio is typically higher and as a result the pH of the substrate increases. This becomes important in understanding that a well formulated hydroponic nutrient contains an ideal ratio of ammonium nitrogen to nitrate nitrogen in order to minimize this situation and better maintain pH stability in the root zone and nutrient solution.
As a general rule, daylight photosynthesis (when the plant is taking up high degrees of mineral nutrition) produces hydrogen ions which can cause the nutrient acidity to increase (lowering the pH). When the lights switch off photosynthesis stops and the plants increase their rate of respiration. This coupled with the respiration of microorganisms (the release of CO2 by microorganisms) uses up the hydrogen ions so the acidity of the solution tends to decrease (pH rises). Additionally, plants are known to release organic acids through their roots (root exudates), reducing pH.
Nutrient Availability and pH
This is an area that tends to be misunderstood and/or oversimplified by many hydroponic industry interests who express optimum pH as 5.5 – 5.8.
In fact, from a scientific perspective, provided that adequate nutrients are available in solution, the acceptable pH range can be expressed as somewhat wider.
That is, the recommended pH for hydroponic growing is specified by many hydroponic nutrient manufacturers/suppliers at 5.5 to 5.8 because overall availability of nutrients is optimized at a slightly acid pH. The availability of Mn, Cu, Zn and especially Fe are reduced at higher pH, and there is a small decrease in availability of P, K, Ca, Mg at lower pH. Reduced availability means reduced nutrient uptake, but not necessarily a nutrient deficiency.
As such, there is some tolerance regarding pH where nutrients don’t become a limiting factor. This is because the direct effects of pH on root growth are small… the problem is reduced nutrient availability at high and low pH.
The pH of a solution can influence the availability of the individual ions within that solution. As pH changes one particular nutrient ion may gradually become more insoluble, leaving less of that ion available to act as a nutrient. pH is of little influence over a range, but if it goes too far, especially too high, then problems can result. Therefore, pH where nutrients are present at adequate levels is less critical than many think.
For example, where hydroponic techniques are used to study the growth of various species apparently preferring different pH levels, researchers usually find that they do reasonably well over a fairly wide pH range (approx. pH 5.2 to 7.5 provided a chelated form of iron is used).
The real issue is in ensuring that enough of any particular ion is in solution at a given pH to cater for the plants nutritional requirements. For example, indoor plants tend to grow equally well between pH 5.2 and 6.3 if nutrients in solution do not become a limiting factor.
Put simply, there is some tolerance to pH where adequate nutrients are available. Therefore, while pH 5.5 – 5.8 is expressed by some as the ideal there is some tolerance with regards to pH and you will typically find that a pH of between 5.2 and 6.3 will perform equally well in indoor settings where nutrients are supplied at adequate levels.
Given the rather confusing scientific understanding surrounding pH you can perhaps understand why many hydroponic and nutrient manufacturers simplify the subject and inform their consumers that they should maintain pH between 5.5 – 5.8. However, it is also necessary to raise the point that this is a simplified version of understanding pH because you will find that some express optimum pH between 5.5 – 5.8 while others express a wider range (e.g. 5.2 – 6.3 or 5.2 – 6.5 etc). This can lead to confusion amongst hydroponic retail consumers because the information provided by one nutrient supplier may seem contradictory to information being provided by another supplier. Additionally, you will find growers give what appears to be conflicting advice on forums with some stating that their plants grow best at e.g. pH 5.5 to 5.8 while others may state that the ideal pH range is wider. However, both versions of optimal/ideal/acceptable pH ranges are, in fact, correct. It really comes down to the fact that nutrient status, nutrient availability and pH are interrelated.
To put things simply, for novice growers, if you strive to maintain pH between the ideals of 5.5 – 5.8 this caters more adequately in situations where nutrients may be a limiting factor. This said, pH tolerance is wider than this in situations where adequate nutrient levels are maintained in solution at all times.
See following image that shows each nutrient’s pH range in hydroponics.
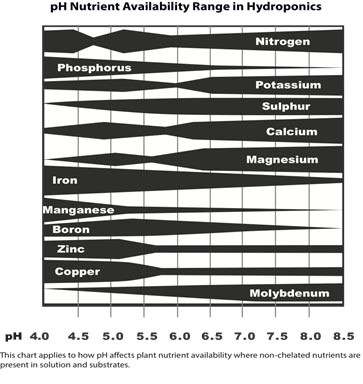
.
The Essential Nutrients and their pH Ranges
Nitrogen (N)
Nitrogen is the plant nutrient which most influences growth and development of agricultural crops. Yield is closely related to N nutrition. Plants are surrounded by nitrogen in the atmosphere, but because atmospheric gaseous nitrogen is present as inert nitrogen (N2) molecules, this nitrogen is not directly available to the plants. Plant available forms of nitrogen in hydroponics are typically inorganic and include nitrate (NO3), and ammonium (NH4). Organic forms of nitrogen that are plant available, which are found in some nutrients and additives are N containing amino acids (e.g. glycine) and the organic chemically pure, albeit, synthetic urea. Nitrogen is available across a wide range of pH values from 2 – 7.
Potassium (K)
There is an extremely important relationship between potassium and nitrogen in flowering/fruiting crops and although potassium is not a constituent of any plant structures or compounds it plays a part in many important regulatory roles in the plant. These include osmotic regulation, regulation of plant stomata and water use, translocation of sugars and formation of carbohydrates, energy status of the plant, the regulation of enzyme activities, protein synthesis and many other processes needed to sustain plant growth and reproduction. Additionally, potassium plays a very important role in plant tolerance of biotic and abiotic stresses.
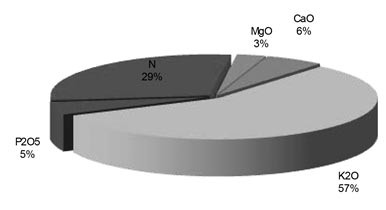
Potassium is also known as the quality nutrient because of its important effects on quality factors (e.g. essential oils, flavonoids). With the exception of nitrogen, potassium is required by plants in much greater amounts than all the other nutrients. Increasing plant vegetative growth, yield as well as fruit quality and chemical composition due to increasing potassium fertilization levels has been reported by many researchers on different crops. The most prevalent nutrient found in the developed tomato plant and fruit is potassium, followed by nitrogen (N) and calcium (Ca). See graphs 1 and 2.
Potassium is almost completely present as a free ion (K+) in a nutrient solution and is available over a wide range of pH values from 2 to 9.
Graph 1: Element composition of a tomato plant (Atherton and Rudich, 1986)
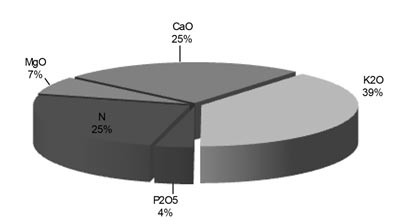
Graph 2: Element composition of a tomato fruit (Atherton and Rudich, 1986)
Calcium and Magnesium (Ca and Mg)
Like nitrogen and potassium, calcium and magnesium are available to plants across a wide range of pH; however, the presence of other ions can interfere with their availability due to the formation of compounds with different grade of solubility. For example, when the pH of the nutrient solution increases, the HPO42– (hydrogen phosphate) ion predominates, which precipitates with Ca2+when the product of the concentration of these ions is greater than 2.2, expressed in mol m-3 . Sulphate also forms relatively strong complexes with Ca2+ and Mg2+. As pH increases from 2 to 9, the amount of SO42-, forming soluble complexes with Mg2+as MgSO4 and with K+ as KSO4– increases. Practically speaking, where hydroponics is concerned, both calcium and magnesium tend to be reasonably plant available between pH 5.5 – 6.0.
Phosphorus (P)
Phosphorus (P) is an important plant macronutrient, making up about 0.2% of a plant’s dry weight. It is a component of key molecules such as nucleic acids, phospholipids, and ATP, and consequently plants cannot grow without a reliable supply of this nutrient. Phosphorus is also involved in controlling key enzyme reactions and in the regulation of metabolic pathways.
Phosphorus is an element which occurs in forms that are strongly dependent on pH. In the root zone phosphorus can be found as PO43-, HPO42, and H2PO4- ions; the last two ions are the main forms of P taken by plants. In inert substrates, the largest amount of P available in a nutrient solution is presented when its pH is slightly acidic (pH 5). In alkaline and highly acidic solutions the concentration of P decreases in a significant way. Namely, with pH 5, 100% of P is present as H2PO4-; this form converts into HPO4-2 at pH 7.3, reaching 100% at pH 10. The pH range that dominates the ion H2PO4-2 on HPO4- is between 5 and 6. In research surrounding P availability by Jacek Dysko et al (2008) with tomatoes grown in various hydroponic organic and inorganic substrates it was shown that regardless of the substrate type, optimum yields were gained at pH 5.5.
“The marketable yield obtained with a pH of 5.5 was significantly higher in relation to the yield obtained at pH 6.5, but it did not differ significantly from the yields obtained at pH 4.5, 5.0 and 6.0. “ Similar findings were made by Chohura et al (2004) while studying the effects of pH in tomato culture grown in rockwool.
Therefore, optimum phosphorus availability in solution and substrates falls within the range of pH 5.0 – 6.0, with pH 5.5 being ideal.
Sulphur (S)
Sulphur is used mainly in sulphur-containing proteins using the amino acids cysteine and methionine. The vitamins thiamine and biotin, as well as the cofactor Coenzyme A, all use sulphur, and so this element also plays a key role in plant metabolism. Sulphur is most available to plants grown hydroponically over a range of 6.0 to 9.5; however because of the availability of other nutrient elements and their pH ranges, sulphur in hydroponics is absorbed reasonably well between pH 5.5 – 6.0.
The Microelements (Fe, Cu, Zn, B, Mn and Mo)
The microelements, iron (Fe), copper (Cu), zinc (Zn), boron (B), molybdenum (Mo) and manganese (Mn), become unavailable in most cases at pH higher than 6.5 and are most available in hydroponic solution at an acidic pH o
Understanding PH & Hydroponics | by Manic Botanix
PH stands for the power of hydrogen, although many refer to the meaning of pH as ‘potential hydrogen’. Either way, potential hydrogen or power of hydrogen, pH is a parameter that measures the acidity or alkalinity of a solution by measuring the hydrogen ion concentration in solution. A pH value indicates the relationship between the concentration of free ions H+ (hydrogen) and OH- (hydroxide) present in a solution. Put simply, if a solution is very acidic, there will be lots of active hydrogen ions and hardly any hydroxide ions. If a solution is very alkaline, the opposite is true. In pure water, the concentrations of hydrogen and hydroxide ions are about the same. Therefore, pure water has a pH that is neutral at pH 7.0.
The pH scale is a logarithmic scale that typically runs from 1 to 14. Each whole pH value below 7 (the pH of pure water) is ten times more acidic than the higher value and each whole pH value above 7 is ten times less acidic than the one below it. For example, a pH of 5 is ten times more acidic than a pH of 6 and 100 times (10 times 10) more acidic than a pH value of 7. So, a strong acid may have a pH of 1-2, while a strong base may have a pH of 13-14. See following illustration of the pH scale.
Why does pH change in nutrient solutions?
This comes back to understanding the power of hydrogen, otherwise known as potential hydrogen in solution and some of our earlier material on EC where we touched on positively charged cations and negatively charged anions in hydroponic solutions. Other than this, pH changes largely occur due to the principle of electroneutrality where chemical reactions take place on an equivalent basis. The law of electroneutrality states that in any single ionic solution (e.g. a hydroponic nutrient solution) a sum of negative electrical charges attracts an equal sum of positive electrical charges. Therefore, according to the principle of electroneutrality, the total charge of an aqueous solution must be zero. For this to occur, the number of positive charges contributed by cations must be equal to the number of negative charges contributed by anions.
Based on this, in very simple terms, when a plant removes a positively charged cation from the nutrient reservoir/tank it leaves a negatively charged anion in its place and when a plant removes an anion from the nutrient reservoir/tank it leaves a cation in its place. See following images.
Since every macro and micro element ion in solution has an electrical charge plants can’t just take them, otherwise the electrical equilibrium would be out of balance. What plants do is swap them with equivalent amounts of H+ and OH- ions.
For example:
Ammonium N (NH4+) is swapped with 1xH+
Nitrate N (NO3-) is swapped with 1xOH-
Potassium (K+) is swapped with 1xH+
Calcium (Ca++) is swapped with 2xH+
Magnesium (Mg++) is swapped with 2xH+
Iron (Fe++) is swapped with 2xH+
Manganese (Mn++) is swapped with 2xH+
Zinc (Zn++) is swapped with 2xH+
phosphates (HPO4–) is swapped with 2xOH-
In this way electrical charge equilibrium remains the same.
The ratio in uptake of anions and cations by plants may cause substantial shifts in pH. In general, an excess of cation over anion leads to a decrease in pH, whereas an excess of anion over cation uptake leads to an increase in pH. That is, when the anions are uptaken in higher concentrations than cations the plant excretes OH- or HCO3- anions to balance the electrical charges inside, which increases the pH value. For example, if a plant absorbs the negatively charged nitrate nitrogen (NO3-) heavily it will start to contribute more OH – than H3O + ions into the solution and the result will be an increase in pH. On the other hand, if the plant absorbs high levels of the positively charged potassium (K+) it will contribute more H3O + than OH – ions and the result will be a decrease in pH.
This phenomenom is frequently seen where plants are grown with a full spectrum nutrient solution that contains nitrogen either as ammonium nitrogen (NH4+) or nitrate (NO3– ) nitrogen. When plants are fed only with NH4+, cation uptake generally exceeds anion uptake and the pH of the substrate decreases. On the other hand, when the plant is fed only with NO3– the uptake of anion to cation ratio is typically higher and as a result the pH of the substrate increases. This becomes important in understanding that a well formulated hydroponic nutrient contains an ideal ratio of ammonium nitrogen to nitrate nitrogen in order to minimize this situation and better maintain pH stability in the root zone and nutrient solution.
As a general rule, daylight photosynthesis (when the plant is taking up high degrees of mineral nutrition) produces hydrogen ions which can cause the nutrient acidity to increase (lowering the pH). When the lights switch off photosynthesis stops and the plants increase their rate of respiration. This coupled with the respiration of microorganisms (the release of CO2 by microorganisms) uses up the hydrogen ions so the acidity of the solution tends to decrease (pH rises). Additionally, plants are known to release organic acids through their roots (root exudates), reducing pH.
Nutrient Availability and pH
This is an area that tends to be misunderstood and/or oversimplified by many hydroponic industry interests who express optimum pH as 5.5 – 5.8.
In fact, from a scientific perspective, provided that adequate nutrients are available in solution, the acceptable pH range can be expressed as somewhat wider.
That is, the recommended pH for hydroponic growing is specified by many hydroponic nutrient manufacturers/suppliers at 5.5 to 5.8 because overall availability of nutrients is optimized at a slightly acid pH. The availability of Mn, Cu, Zn and especially Fe are reduced at higher pH, and there is a small decrease in availability of P, K, Ca, Mg at lower pH. Reduced availability means reduced nutrient uptake, but not necessarily a nutrient deficiency.
As such, there is some tolerance regarding pH where nutrients don’t become a limiting factor. This is because the direct effects of pH on root growth are small… the problem is reduced nutrient availability at high and low pH.
The pH of a solution can influence the availability of the individual ions within that solution. As pH changes one particular nutrient ion may gradually become more insoluble, leaving less of that ion available to act as a nutrient. pH is of little influence over a range, but if it goes too far, especially too high, then problems can result. Therefore, pH where nutrients are present at adequate levels is less critical than many think.
For example, where hydroponic techniques are used to study the growth of various species apparently preferring different pH levels, researchers usually find that they do reasonably well over a fairly wide pH range (approx. pH 5.2 to 7.5 provided a chelated form of iron is used).
The real issue is in ensuring that enough of any particular ion is in solution at a given pH to cater for the plants nutritional requirements. For example, indoor plants tend to grow equally well between pH 5.2 and 6.3 if nutrients in solution do not become a limiting factor.
Put simply, there is some tolerance to pH where adequate nutrients are available. Therefore, while pH 5.5 – 5.8 is expressed by some as the ideal there is some tolerance with regards to pH and you will typically find that a pH of between 5.2 and 6.3 will perform equally well in indoor settings where nutrients are supplied at adequate levels.
Given the rather confusing scientific understanding surrounding pH you can perhaps understand why many hydroponic and nutrient manufacturers simplify the subject and inform their consumers that they should maintain pH between 5.5 – 5.8. However, it is also necessary to raise the point that this is a simplified version of understanding pH because you will find that some express optimum pH between 5.5 – 5.8 while others express a wider range (e.g. 5.2 – 6.3 or 5.2 – 6.5 etc). This can lead to confusion amongst hydroponic retail consumers because the information provided by one nutrient supplier may seem contradictory to information being provided by another supplier. Additionally, you will find growers give what appears to be conflicting advice on forums with some stating that their plants grow best at e.g. pH 5.5 to 5.8 while others may state that the ideal pH range is wider. However, both versions of optimal/ideal/acceptable pH ranges are, in fact, correct. It really comes down to the fact that nutrient status, nutrient availability and pH are interrelated.
To put things simply, for novice growers, if you strive to maintain pH between the ideals of 5.5 – 5.8 this caters more adequately in situations where nutrients may be a limiting factor. This said, pH tolerance is wider than this in situations where adequate nutrient levels are maintained in solution at all times.
See following image that shows each nutrient’s pH range in hydroponics.
.
The Essential Nutrients and their pH Ranges
Nitrogen (N)
Nitrogen is the plant nutrient which most influences growth and development of agricultural crops. Yield is closely related to N nutrition. Plants are surrounded by nitrogen in the atmosphere, but because atmospheric gaseous nitrogen is present as inert nitrogen (N2) molecules, this nitrogen is not directly available to the plants. Plant available forms of nitrogen in hydroponics are typically inorganic and include nitrate (NO3), and ammonium (NH4). Organic forms of nitrogen that are plant available, which are found in some nutrients and additives are N containing amino acids (e.g. glycine) and the organic chemically pure, albeit, synthetic urea. Nitrogen is available across a wide range of pH values from 2 – 7.
Potassium (K)
There is an extremely important relationship between potassium and nitrogen in flowering/fruiting crops and although potassium is not a constituent of any plant structures or compounds it plays a part in many important regulatory roles in the plant. These include osmotic regulation, regulation of plant stomata and water use, translocation of sugars and formation of carbohydrates, energy status of the plant, the regulation of enzyme activities, protein synthesis and many other processes needed to sustain plant growth and reproduction. Additionally, potassium plays a very important role in plant tolerance of biotic and abiotic stresses.
Potassium is also known as the quality nutrient because of its important effects on quality factors (e.g. essential oils, flavonoids). With the exception of nitrogen, potassium is required by plants in much greater amounts than all the other nutrients. Increasing plant vegetative growth, yield as well as fruit quality and chemical composition due to increasing potassium fertilization levels has been reported by many researchers on different crops. The most prevalent nutrient found in the developed tomato plant and fruit is potassium, followed by nitrogen (N) and calcium (Ca). See graphs 1 and 2.
Potassium is almost completely present as a free ion (K+) in a nutrient solution and is available over a wide range of pH values from 2 to 9.
Graph 1: Element composition of a tomato plant (Atherton and Rudich, 1986)
Graph 2: Element composition of a tomato fruit (Atherton and Rudich, 1986)
Calcium and Magnesium (Ca and Mg)
Like nitrogen and potassium, calcium and magnesium are available to plants across a wide range of pH; however, the presence of other ions can interfere with their availability due to the formation of compounds with different grade of solubility. For example, when the pH of the nutrient solution increases, the HPO42– (hydrogen phosphate) ion predominates, which precipitates with Ca2+when the product of the concentration of these ions is greater than 2.2, expressed in mol m-3 . Sulphate also forms relatively strong complexes with Ca2+ and Mg2+. As pH increases from 2 to 9, the amount of SO42-, forming soluble complexes with Mg2+as MgSO4 and with K+ as KSO4– increases. Practically speaking, where hydroponics is concerned, both calcium and magnesium tend to be reasonably plant available between pH 5.5 – 6.0.
Phosphorus (P)
Phosphorus (P) is an important plant macronutrient, making up about 0.2% of a plant’s dry weight. It is a component of key molecules such as nucleic acids, phospholipids, and ATP, and consequently plants cannot grow without a reliable supply of this nutrient. Phosphorus is also involved in controlling key enzyme reactions and in the regulation of metabolic pathways.
Phosphorus is an element which occurs in forms that are strongly dependent on pH. In the root zone phosphorus can be found as PO43-, HPO42, and H2PO4- ions; the last two ions are the main forms of P taken by plants. In inert substrates, the largest amount of P available in a nutrient solution is presented when its pH is slightly acidic (pH 5). In alkaline and highly acidic solutions the concentration of P decreases in a significant way. Namely, with pH 5, 100% of P is present as H2PO4-; this form converts into HPO4-2 at pH 7.3, reaching 100% at pH 10. The pH range that dominates the ion H2PO4-2 on HPO4- is between 5 and 6. In research surrounding P availability by Jacek Dysko et al (2008) with tomatoes grown in various hydroponic organic and inorganic substrates it was shown that regardless of the substrate type, optimum yields were gained at pH 5.5.
“The marketable yield obtained with a pH of 5.5 was significantly higher in relation to the yield obtained at pH 6.5, but it did not differ significantly from the yields obtained at pH 4.5, 5.0 and 6.0. “ Similar findings were made by Chohura et al (2004) while studying the effects of pH in tomato culture grown in rockwool.
Therefore, optimum phosphorus availability in solution and substrates falls within the range of pH 5.0 – 6.0, with pH 5.5 being ideal.
Sulphur (S)
Sulphur is used mainly in sulphur-containing proteins using the amino acids cysteine and methionine. The vitamins thiamine and biotin, as well as the cofactor Coenzyme A, all use sulphur, and so this element also plays a key role in plant metabolism. Sulphur is most available to plants grown hydroponically over a range of 6.0 to 9.5; however because of the availability of other nutrient elements and their pH ranges, sulphur in hydroponics is absorbed reasonably well between pH 5.5 – 6.0.
The Microelements (Fe, Cu, Zn, B, Mn and Mo)
The microelements, iron (Fe), copper (Cu), zinc (Zn), boron (B), molybdenum (Mo) and manganese (Mn), become unavailable in most cases at pH higher than 6.5 and are most available in hydroponic solution at an acidic pH o
Mountainman80
Well-Known Member
To.lower pH use cider vinegar to raise it lime waterA blast from the Past
Will my nitrogen source affect the PH?
Contributed by: diels alder
The source of nitrogen in your fertilizer affects the pH of the medium you are growing in. The standard of measure of how acidic/basic a source is, is calcium carbonate, a common water mineral. Calcium carbonate is the major contributor of water alkalinity, the capacity for water to 'soak up', or buffer, acidity in water and lead to a high pH.
Note that it is much easier to lower the pH of water through the acidity of a given nitrogen source than it is to raise it, as nitrates are less basic than ammonium is acidic.
code:--------------------------------------------------------------------------------
N Source Potential acidity Potential Basicity
Ammonium sulfate 2,200 0
Urea 1,680 0
Diammonium phosphate 1,400 0
Ammonium nitrate 1,220 0
Monoammonium phosphate 1,120 0
Calcium nitrate 0 400
Potassium nitrate 0 520
Sodium nitrate 0 580
--------------------------------------------------------------------------------
Potential acidity = # of lbs of calcium carbonate needed to neutralize acidity of one ton of source.
Potential basicity = one ton of source has same effect as this many lbs of calcium carbonate.
Source:GrowFAQ © 2000-2004 Overgrow
I wouldn't...in a pinch maybe on a soil grow (ive no clue).To.lower pH use cider vinegar to raise it lime water
Put that into an RDWC- and you'll wreck havoc.
Also- the quote you tagged is from 2009...happens to all of us.
Mountainman80
Well-Known Member
Didn't realize you were doing rdwc pH up pH down be ideal for uI wouldn't...in a pinch maybe on a soil grow (ive no clue).
Put that into an RDWC- and you'll wreck havoc.
Also- the quote you tagged is from 2009...happens to all of us.
zigzagman1960
Well-Known Member
I NEVER check pH, indoor or outdoor. Jacks 20 20 20 once a week in veg. Jacks 10 30 20 twice a week in flower. No ec measurements nothing fancy. Cal mag every feeding at 5ml/gal


Mountainman80
Well-Known Member
There looking awesome huge colasI NEVER check pH, indoor or outdoor. Jacks 20 20 20 once a week in veg. Jacks 10 30 20 twice a week in flower. No ec measurements nothing fancy. Cal mag every feeding at 5ml/gal

Anyone familiar with the Bluelabs pH Pen?? I just got more calibration solution in. Went to recalibrate pen, worked perfectly with the pH 7.0 solution. But I keep getting an Er message when I try to recalibrate in the 4.0Solution.... I have no idea why or how this is happening. Any help would be AWESOME!?!I was going to put this in a problem thread but feel as though it will get better visibility here. So I'll keep here...
pH is extremely important and one of the most often overlooked parameters by new growers. I know because I also overlooked and underestimated the importance of pH when I started growing.
In soil the pH must be between 6.3 and 6.7. Period well + or - .1 some will argue. This is a very finite range. You will find out it does not take much to skew pH too much in either direction. many soil growers battle to keep pH up at acceptable levels while many hydro growers fight to keep the pH down to acceptable levels.
Why pH tends to go low in soil...
Most soil / soilless mediums are comprised of some failry large percentage of sphagnum moss. As this moss degrades and breaks down it becomes very acidic thus effecting nutrient uptake by way of lockout. Certain nutrients/elements get locked out at either too low or too high of a pH. This is noticable by plant show extreme deficiencies of one or more nutrients when you are feeding the plant all nutrients and the leaves may also look crumpled or twisted.
Best way to counter this in soil is to "sweeten" the soil by adding pulverized dolomitic lime which is very alkaline. A little bit goes a long way. It will help keep the pH stable offsetting the acidic breakdown of the moss.
Why pH tends to go HIGH in hydro...
Water with nothing in it is pH neutral. or pH of 7.0
Adding your hydroponic nutrients to the water will tend to bring the pH down. The nutrients them selves have acidic properties. Either naturally or are made this way by the nutrient manufacturer to help get your reservoir to an acceptable pH range. In hydro it tend to be a negative pH shift of about .7 of soil acceptable range. so 5.5 -6.2 or so. In hydro it is beneficial to flux within the range to best absorb all nutrients. So set res at 5.5 let flux to 6.2 then bring back to 5.5 etc.
As the plant absorb the nutrients (remember which are acidic and lowering the pH) from the water the pH will shift north towards neutral.
The more plants you have and the less reservoir space you have will lead to huge fluctuations in a short period of time. Unless you want to stand over your reservoir constantly monitoring and adjusting for pH and PPM then you should plan on 5 gallons or more of reservoir volume per plant.
I use a Bluelabs soil pH Pen.So what is everyone using for a PH meter?
Hugoi
420 Member
Thank you @RacefanWhat is pH?
pH is one of the most common analyses in soil and water testing. An indication of the sample’s acidity, pH is actually a measurement of the activity of hydrogen ions in the sample.
pH measurements run on a scale from 0-14, with 7.0 considered neutral. Those solutions with a pH below 7.0 are considered acids, and those above 7.0 are designated bases. The pH scale is logarithmic, so a one unit change in pH actually reflects a ten-fold change in the acidity. For instance, orange juice (pH 4) is ten times more acidic than cottage cheese, which has a pH of 5.
Many industries rely heavily on pH for their processes to work properly, or to maintain expensive equipment. Breweries maintain the pH between 4.2 and 4.6 to keep infectious bacteria from breeding during the fermentation process. In many industrial applications, if the pH is too low the water may corrode metal equipment, but if it is too high scaling may result.
pH can be measured visually or electronically. Visual comparisons use a pH indicator whose color change reflects the pH, which is then matched to a color
standard. pH meters, such as the pH 5, simplify the pH test. A probe is placed in
the sample, and the pH is read directly from the meter.
While the meter is very easy to use, the electronics within the meter are more
complex. After the pH probe measures the millivolts of potential between the
reference electrode and the pH electrode, the meter converts this reading to pH
units using the Nernst Equation:
where Ex = constant depending upon reference electrode
R= constant
Tk = absolute temperature
n = charge of the ion (including sign)
F = constant
ai = activity of the ion
Electrode Cleaning
Because your pH electrode is susceptible to dirt and contamination, clean it
every one to three months depending on extent and condition of use.
Clean the electrode in a mild detergent solution. Wipe the probe with a soft
tissue paper. Avoid touching the glass membrane with your fingers. Rinse
thoroughly in tap water and then in distilled water. Recalibrate your meter after
cleaning the electrode.
Storage
The pH electrode should always be stored in the soaker bottle. The cap should
be tightened to prevent leaks. The soaker bottle contains a dilute solution of
potassium chloride.
Special Cleaning Tips
Salt deposit: dissolve the deposit by immersing the electrode in tap water for ten
to fifteen minutes. Then thoroughly rinse with distilled water.
Oil/grease film: wash electrode pH bulb gently in detergent solution. Rinse
electrode tip with distilled water.
Clogged reference junction: heat a diluted KC1 solution to 60-80°C. Place the
sensing part of the electrode into the heated solution for about 10 minutes.
Allow the electrode to cool in some unheated KC1 solution.
Protein deposits: prepare a 1% pepsin solution in 0.1M of HC1. Place the
electrode in the solution for five to ten minutes. Rinse the electrode with
distilled water.
 for explanation on pH It is really helpful; I appreciate the cleaning and storage tips for the pH electrode as well. Keeping the equipment in good condition is crucial; I am exited to applying this knowledge to my indoor growing setup.
for explanation on pH It is really helpful; I appreciate the cleaning and storage tips for the pH electrode as well. Keeping the equipment in good condition is crucial; I am exited to applying this knowledge to my indoor growing setup.


