~ DIY CALMAG TESTS ~
Here are the truly yawntastic results of the tests I've been doing on my DIY calmag concoctions.
I did all sorts of mixes, and which have been clogging my shelves for the last while. I'd let them sit for a few days and test the pH. Then I made a bunch more mixes and tested the pH of those. I decided against creating some sort of elaborate table of information. It would be more 'scientificy' but probably not accurate enough, due to sloppy lab practices and other variables, to justify all the extra work. Mostly all I need to know is the basic trends.
I made three basic DIY calmag mixes.
For the purposes of these latest tests I made a ph balanced medium strength (500 ppm) solution of my regular grow feed nutrient.
Ph of the nute mix was at 5.6 before adding the calmag stuff.
I tried many variations with molasses and Epsom salts and many combinations of the basic ingredients, and tests using different amounts of each, to see if there were any weird ph-related reactions between two or more of the ingredients. I didn't find anything too dramatic there though. Basically I found the same trends as occurred when I first fed these mixes to my DTW plants. So I'll pretty much skip all the scientificy test results and tell you what happened when I fed them to the plants. Here's a pillow - Just lay your head down there, yes. Zzzzzzz
~ DIY CALMAG 1 ~
This was the first one I added to the hex plants. It was made by mixing calcium carbonate and Epsom salts, at about a 2:1 ratio, in water. The calcium was bought from a drugstore in the vitamin/mineral section.
Calcium carbonate, or CaCO3, comprises more than 4% of the earth's crust and is found throughout the world. -Its most common natural forms are chalk, limestone, and marble, produced by the sedimentation of the shells of small fossilized snails, shellfish, and coral over millions of years. -Although all three forms are identical in chemical terms, they differ in many other respects, including purity, whiteness, thickness and homogeneity. -Calcium carbonate is one of the most useful and versatile materials known to man.
Many of us encounter calcium carbonate for the first time in the school classroom, where we use blackboard chalk. -Chalk has been used as a writing tool for over 10,000 years and is a fine, microcrystalline material. -As limestone, calcium carbonate is a biogenic rock, and is more compacted than chalk. -As marble, calcium carbonate is a coarse-crystalline, metamorphic rock.
There's more- and it's very interesting. But basically - we are talking about chalk/limestone. My advice is not to buy calcium supplements from the drugstore. Go back to school instead. You'll learn stuff like this, and have all the calcium supplements you need thrown at your head by the teacher.
Lime is something we add to our garden soil to regulate ph at around 7 and, for those smart pupils out there- smarter than me apparently, this gives a great clue as to how my DIY calmag affects ph.
I added this to the nute mix, at a rate of about 150 ppm. It seemed ok at first so I left it - but by the next day the mix was up to 7 ph (ding ding -very bad!) where it stubbornly tried to stay despite my pouring in large amounts of ph down.
*A note about this calcium/Epsom mix. I made a large jug of it. A few days later it was smelling absolutely Putrud.
A few days after that it was Horrifically Putrid and I threw the jug far away from me. I guess calcium rots, apparently.
~ DIY CALMAG 2 ~.
This one was made with milk and molasses. I did not add it directly to the reservoir as that seemed a bad idea. I just fed it to the plants and saved the rest of the batch for testing.
I used the milk at Tead's recommended strength of 2 tsp/gallon. Though I did notice that the milk didn't raise the ppm much, and to approach the levels I would normally add with the store-bought calmag, I would have needed to add quite a bit more. Molasses I added at 5ml/gallon.
The milk seemed to raise the ph by a little bit, but not enough to bother me.
At this point I went away for a few days.
Upon return I found the ph to be at 4:3 (alarm bells!!!!) The plants didn't look real excited about their little acid trip either.
- I spent a lot of time in my science lab testing milk, in different amounts and with different mixes- plain, with molasses, with Epsom salts, etc. But here's the gist of it (if you'll wake up long enough to listen).
Milk has a buffering quality, like other calcium sources, as we (maybe) know- and it seems like something you might drink for an upset stomach. So yeah- when I add it to a nute solution it tends to want to raise it a bit. Probably if I added enough it would raise it up close to 7 or at least the high sixes.
BUT- then it starts to fall. All my tests showed this. It would fall down as low as 4:1 in some cases. That's horribly low.
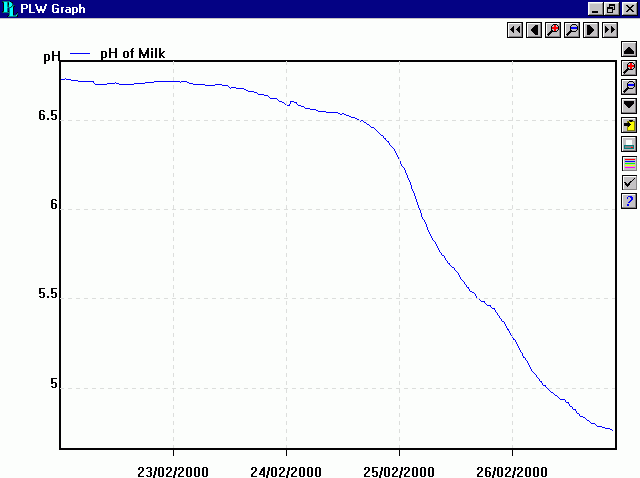
Last night I did some googling and confirmed this. Milk gets acidic - as it sours.
What's more - I stumbled on this graph just this morning- which was made by actual real scientists- and shows exactly the results I've been getting! I am surprised and conclude that I have a great science career ahead of me.
~ DIY CALMAG 3 ~
For this one I decided to get a bit more serious and try something that has already been tried and tested somewhat. I tried Emmie's DIY calmag from her thread here-
Emmie's DIY CalMagPhos+ From Eggshells
Lacking eggshells I used oyster shells which I baked slowly in my wood stove and then crushed and ground into powder. Then the powder was dissolved in vinegar at a 1/8 ratio. I'm pretty sure it's dissolved to the saturation point as there's plenty left on the bottom of the jar still.
Ph of this mix seems to be slowly rising as it was at 5.2 last week and now is at 5.7
Still- seems like a good ph.
I added this to my reservoir, along with 5ml/gallon of Epsom salts.
Results were similar to mix #1. I came back the next day to find my babies getting fed at 6.7. Very unimpressed, they were. (And still are)
Basically - the calcium in this mix is doing its best to raise the ph to 7. Not sure what happened to the vinegar that was keeping it in check. All my test mixes were up in the high 6s after a few days.
~ A FEW MORE NOTES ~
- Molasses- this lowers ph, but not by a huge amount.
- Epsom salts. Very little effect on ph but it's crazy strong and raises ppm drastically. I'm thinking that 5ml/gallon is way too much.
~ CONCLUSION ~
Stay in school, kids. You'll be able to get a good job and afford to buy proper calmag, you teeth and bones will be strong and shiny from chalk dust inhalation, and your plants won't look like mine.
Wake up!





 I can see you read this in front of class, just the way you wrote it, excellent and funny.
I can see you read this in front of class, just the way you wrote it, excellent and funny.




