Cannabis use improves retention and virological
outcomes in patients treated for hepatitis C
Eur J Gastroenterol Hepatol Oct 2006 18:1057-1063
Diana L. Sylvestrea,b, Barry J. Clementsb and Yvonne Malibub
aDepartment of Medicine, University of California, San Francisco, California, USA
and bOrganization to Achieve Solutions in Substance-Abuse (OASIS), Oakland, California, USA
Note from Jules Levin: this study was first presented at a conference 2 years ago or longer.
“…The results of this observational study suggest that the use of cannabis during HCV treatment can improve adherence by increasing the duration of time that patients remain on therapy; this translates to reduced rates of post-treatment virological relapse and improved SVR…..”
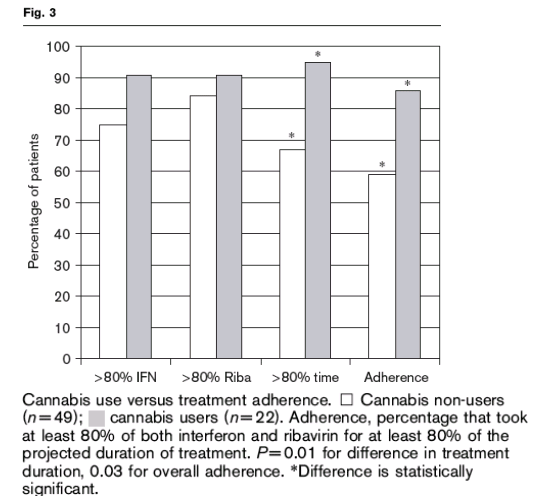
As shown in Fig. 3, cannabis users were no more likely than non-users to take at least 80% of the prescribed interferon, 91 versus 76% (P=0.2), nor were they more likely to take at least 80% of the prescribed ribavirin, 91 versus 84% (P>0.5). However, cannabis users were significantly more likely than non-users to remain on HCV treatment for at least 80% of the projected treatment course, 95 versus 67% (P=0.01). The average duration of HCV treatment in cannabis users was 38 weeks compared with 33 weeks for the non-users.
ABSTRACT
Objectives: Despite the widespread use of polypharmacy, the management of hepatitis C virus (HCV) treatment-related side-effects is often incomplete, and many patients turn to cannabis for symptom relief. Unfortunately, there are few data about cannabis use on treatment outcomes, leaving clinicians without the data needed to inform recommendations.
Methods: To define the impact of cannabis use during HCV treatment, we conducted a prospective observational study of standard interferon and ribavirin treatment in 71 recovering substance users, of whom 22 (31%) used cannabis and 49 (69%) did not.
Results:
Seventeen of the 71 study patients (24%) discontinued therapy early, one cannabis user (5%) and 16 non-users (33%) (P = 0.01).
Overall, 37 patients (52%) were end-of-treatment responders, 14 (64%) cannabis users and 23 (47%) non-users (P =0.21).
A total of 21 out of 71 (30%) had a sustained virological response: 12 of the 22 cannabis users (54%) and nine of the 49 non-users (18%) (P =0.009), corresponding to a post-treatment virological relapse rate of 14% in the cannabis users and 61% in the non-users (P = 0.009).
Overall, 48 (68%) were adherent, 29 (59%) non-users and 19 (86%) cannabis users (P =0.03).
Although cannabis users were no more likely than non-users to take at least 80% of the prescribed interferon or ribavirin, they were significantly more likely to remain on HCV treatment for at least 80% of the projected treatment duration, 95 versus 67% (P =0.01).
Conclusions: Our results suggest that modest cannabis use may offer symptomatic and virological benefit to some patients undergoing HCV treatment by helping them maintain adherence to the challenging medication regimen.
Introduction
Although hepatitis C virus (HCV) treatment outcomes have improved dramatically over the past decade, the intolerability of interferon/ribavirin combination therapy remains a barrier to treatment success. The majority of patients develop significant treatment-related side effects [1-5], with almost 80% experiencing an initial ‘flulike syndrome that includes fevers, chills, and muscle and joint aches. Although the acute effects of treatment tend to modulate over time, many will experience debilitating fatigue (70-72%), headaches (66-67%), nausea (35-46%), anorexia (19-27%), depression (21-44%), and insomnia (30-39%) among others [3,5-7].
Many patients require the use of adjunctive pharmacological agents for side-effect management [5,8]. These include a spectrum of medications including antiemetics, anti-inflammatory agents, antihistamines, sleeping pills, antidepressants, anxiolytics, stimulants, and antipsychotics. Unfortunately, symptom relief is often incomplete despite the widespread use of polypharmacy, and patients so affected may compensate by reducing their interferon or ribavirin doses or by discontinuing treatment altogether. Maximizing HCV treatment outcomes thus requires a thorough familiarity with an array of successful side-effect management strategies [5,9,10].
Faced with intolerable treatment-related side-effects that respond inadequately to conventional medications, some patients turn to Cannabis sativa (marijuana) for symptom relief. Cannabis sativa contains over 400 chemical entities [11,12], but delta-9-tetrahydrocannabinol (THC) is the major psychoactive component. Although the majority of studies of cannabis are observational in nature, there is anecdotal evidence that it may have benefits in modulating some of the common side-effects associated with HCV treatment, including nausea [13,14], anorexia [15,16], weight loss [17], musculoskeletal pain [18-21], insomnia [22], anxiety [23], and mood instability [24]. However, the benefits of cannabis during HCV treatment remain unconfirmed and concerns about its safety remain [25-27]. Cannabinoid receptors appear to be upregulated in hepatic myofibroblasts of human cirrhotic liver samples [28], and smoking daily cannabis has been reported to accelerate the progression of hepatic fibrosis in patients with chronic HCV [29]. Cannabinoid receptors are also present on immune cells [30], and cannabis use may suppress a variety of immune functions, including antibody production [31], cell proliferation [32], natural killer cell activity [33], and macrophage function [34,35], and also alter the production of such cytokines as interferon gamma and tumor necrosis factor [36]. In addition, there is a potential drug-drug interaction between ribavirin and marijuana, as both are metabolized by the cytochrome P450 system [37]. Obviously, the overall benefit of cannabis in terms of side-effect management may be outweighed by worsening histology and impairments in virological outcomes; therefore, its use as a potential therapeutic agent must be more clearly defined in the setting of HCV treatment.
Although widespread restrictions limit the ease with which these questions can be formally studied, the pervasive use of cannabis during HCV treatment provides a means for an observational study of its potential risks and benefits. In the context of a prospective study of HCV treatment in recovering heroin users maintained on methadone we have conducted such a study, by measuring the impact of intercurrent cannabis use on treatment adherence, retention rates, and virological outcomes.
Discussion
The results of this observational study suggest that the use of cannabis during HCV treatment can improve adherence by increasing the duration of time that patients remain on therapy; this translates to reduced rates of post-treatment virological relapse and improved SVR. Although other potential mechanisms may contribute to its enhancement of treatment outcomes, such as altered immunological function and improved nutritional status, it appears that the moderate use of cannabis Although its availability in the United States has been restricted since 1937 and its benefit unconfirmed, cannabis is frequently obtained illicitly for self-medication. It has been used recreationally for millennia, and is the third most commonly used drug after tobacco and alcohol [39]. In the United States, 6.2% of individuals aged 12 years or older have used cannabis in the past month, with 4.8 million individuals using it on 20 or more days [39].
THC can produce alterations in mood, perception,cognition, and memory [14], and studies have shown that THC has anticonvulsive, analgesic, anti-anxiety, and anti-emetic properties [13,14]. Clinical trials have demonstrated that cannabinoids reduce nausea and improve appetite in humans [15,16], and cannabis has shown benefit in modulating the nausea of cancer chemotherapy [40-43], multiple sclerosis-related spasticity [44], and the wasting syndrome of HIV [17]. Progress has been made in understanding the pharmacology of cannabinoids in humans. Of the two known cannabinoid receptors, CB1 is responsible for the neurological and behavioral effects of marijuana. CB1 was the first cannabinoid receptor identified [45], and is the most abundant G-protein-coupled receptor in the central nervous system [13]. It is also expressed on peripheral neurons and is found abundantly in the basal ganglia, cerebellum, and hippocampus, accounting for its effects on motor coordination and short-term memory [46]. It is also expressed at high concentrations on primary afferent nociceptors of the dorsal spinal cord, which are responsible for the ability of cannabinoids to inhibit pain [13].
Although CB1 cannabinoid receptors mediate the central nervous system effects of cannabinoids [46], an additional subset of cannabinoid receptors, the CB2 receptors, is present on immune cells [30]. The presence of these receptors on B lymphocytes and natural killer cells suggests that cannabinoids may impact upon the immune response. Some studies have shown that THC can be immunosuppressive and can impair cell-mediated immunity [32,47,48], humoral immunity [49], and cellular defences against a variety of infectious agents in experimental animals [35,47,50]. There is an increased recurrence of herpes simplex viral lesions in marijuana smokers [51] and an altered responsiveness of human papilloma virus to IFN-a 2a treatment [52]. Although uncontrolled studies suggested an association between marijuana use and the progression of HIV disease, a recent prospective study demonstrated no evidence of detrimental effects of cannabinoids on immune parameters in patients with HIV [53]. The majority of studies on the effects of cannabis have been conducted in cell culture or on animal models with supraphysiological doses of the compound, and their clinical relevance is unclear.
Although their potential contribution to liver disease is not understood, both the CB1 and CB2 receptors have also been reported to be expressed on hepatic myofibroblasts in cirrhotic livers [28]. Activation of these receptors can lead to cellular apoptosis, and a recent study demonstrated that the use of cannabis on a daily basis may enhance the progression of hepatitis fibrosis in patients with HCV [29]. By implicating these receptors as mediators of the fibrotic process, these results raise concerns about the safety of cannabis use in patients with HCV.
In spite of this, our results suggest that moderate cannabis use during HCV treatment may offer significant benefit to certain patients. Although the lack of a direct dose response suggests that its principal contribution is related to a non-specific improvement in the tolerability of the challenging medication regimen, we cannot rule out additional biological effects. We did not measure relevant immune parameters in our patients, nor did we assess potential differences in nutritional status. P450-mediated drug-drug interactions between cannabinoids and ribavirin may have led to additional benefit, but these were not assessed. However, the lack of dose response in our study argues against specific receptor or metabolismrelated effects, and suggests instead that cannabis exerted its benefit by non-specific improvements in symptom management. Interestingly, because the bene fits of heavy cannabis use were less apparent, we cannot rule out the possibility that detrimental biological or immunological mechanisms may be relevant at higher levels of consumption. Obviously, further study is needed.
Our study has a number of additional limitations that warrant caution in its interpretation. First, we confined our study to methadone-maintained patients, a population with relatively high rates of medical and psychiatric co-morbidity. Second, the use of additional illicit substances was not uncommon, and although not differing between the two cohorts, the impact of these substances or even of methadone on study outcomes cannot be excluded. Third, the use of marijuana was quantified by self-report and may have introduced bias as a result of underreporting or even overreporting. Fourth, illicitly obtained marijuana, even that obtained through ‘cannabis clubs’, may be highly variable in its content of bioactive compound, leaving in question a true quantitation of the amount of cannabis that may or not be beneficial. And finally, significant limitations are introduced by our observational study design; however, with legal proscriptions against cannabis use limiting its study, the design and conduct of randomized, prospective research studies is virtually impossible at this time.
Despite its shortcomings, this study begins to answer some of the key questions that arise about the use of cannabis during HCV treatment. Our results suggest that the modest use of cannabis does not appear to impact negatively upon HCV treatment outcomes and need not elicit undue alarm. The widespread use of illicit cannabis during HCV therapy highlights the inadequacies of our current side-effect management strategies; our study suggests that cannabis use may offer benefit for some patients undergoing HCV treatment by helping them maintain adherence to the frequently debilitating medication regimen. However, the mechanisms through which cannabis exerts its benefit are unclear, and controlled studies may further elucidate the mechanisms through which cannabis may impact upon clinical outcomes during HCV treatment.
Results
Baseline characteristics
Seventy-one patients were enrolled; 22 (31%) smoked cannabis while undergoing HCV treatment and 49 (69%) did not. The demographic characteristics of the study patients are shown in Table 1. The median age was 50 years, and 43 (61%) were male, 53 (75%) Caucasian, 10 (14%) African-American, and eight (11%) Latino; there were no differences in the demographic characteristics between the cannabis users and non-users. The median estimated duration of HCV exposure was 30±9 years. Forty patients (56%) had genotype 1, 29 (41%) had genotypes 2 or 3, one patient had genotype 8a, and one patient’s genotype was untypable. There was no difference in the frequencies of genotypes between the cannabis users and non-users; 30 of the non-users (61%) and 10 of the cannabis users (48%) had genotype 1 (P=0.31). Thirty patients underwent liver biopsy. Among these, the mean METAVIR inflammation grade was 2.4 (1.5-3.5) and the mean fibrosis stage was 2.6 (0-4). There was no significant difference in liver fibrosis between the two groups; the mean fibrosis stage was 2.5±0.4 for the cannabis users and 2.7±0.2 for the nonusers (P=0.36). The 20 patients (28%) who had platelet counts of less than 100 000 cells/ml were also equally divided between the groups, comprising 29% (n=14) of the non-users and 27% (n=6) of the group that used cannabis.
Forty-two patients (59%) reported a previous psychiatric diagnosis; the majority had depression (n=33) or depression/anxiety (n=6). Cannabis users were no more likely to report a psychiatric diagnosis than non-users (P>0.5), and there were no differences in the rates of antidepressant use between users and non-users during HCV treatment (P>0.5). Similarly, a total of 25 (35%) used other illicit substances during HCV treatment, including heroin, cocaine, and methamphetamine, but this did not differ between the two groups (37% in the cannabis non-users and 32% in the users; P>0.5), nor were there differences in rates of alcohol consumption (24% in the non-users and 14% in the users; P=0.36).
Treatment outcomes
The majority of patients, 93% (n=66), reported at least one treatment-related side-effect, most commonly ‘flulike symptoms, nausea, or headache, but there was no difference in reported symptoms between the cannabis users and non-users (P>0.5). The association of cannabis use with HCV treatment outcomes is shown in Fig. 1. Seventeen of the 71 study patients (24%) discontinued therapy before completing the full course. Of these, 16 did not use cannabis and one was a cannabis user. The discontinuation rate of the 49 cannabis nonusers was 33%; it was 5% in the cannabis users (P=0.01). Of the 16 non-users who terminated treatment early, eight discontinued as a result of intolerable side-effects and four discontinued because of depression. Three of the 16 were terminated at the discretion of the medical provider: one because of excessive alcohol intake, one because of worsening liver disease, and one because of intractable anemia. The remaining patient in this cohort relocated and was unable to obtain medications. The single cannabis user who discontinued treatment developed worsening liver disease and was unable to continue.
Overall, 37 of the 71 patients (52%) were end-of-treatment responders and 21 (30%) had an SVR. The association of cannabis use with response rates is shown in Fig. 1. Fourteen of the cannabis users (64%) and 23 of the non-users (47%) were end-of-treatment responders (P=0.21). Twelve of the 22 cannabis users (54%) and nine of the 49 non-users (18%) had an SVR, corresponding to a post-treatment relapse rate of 14% (n=2) with the cannabis users and 61% (n=14) with the non-users. Multivariate logistic regression analysis taking sex, race, genotype, and the use of other illicit substances into account, revealed that this finding was statistically significant (P=0.009).
The association of the estimated quantity of cannabis used with virological outcomes is shown in Fig. 2. Ten of the 16 occasional cannabis users (62%) had an end-oftreatment virological response compared with four of the six regular users (67%, P>0.5). SVR were also not statistically different between the occasional and regular users of cannabis, seen in two of six of the regular users (33%) and 10 of the 16 (62%) occasional users (P=0.35).
Adherence
The association of cannabis use with the components of treatment adherence is shown in Fig. 3. Overall, 48 of the 71 study patients (68%) took at least 80% of the prescribed interferon and ribavirin for at least 80% of the projected duration of treatment, and were therefore considered adherent. Of those, 29 did not use cannabis and 19 were cannabis users. The corresponding adherence rates were 59% in the non-cannabis group and 86% in the cannabis group (P=0.03); there was no difference in adherence between occasional users (87%) and regular users (83%) (P>0.5).
As shown in Fig. 3, cannabis users were no more likely than non-users to take at least 80% of the prescribed interferon, 91 versus 76% (P=0.2), nor were they more likely to take at least 80% of the prescribed ribavirin, 91 versus 84% (P>0.5). However, cannabis users were significantly more likely than non-users to remain on HCV treatment for at least 80% of the projected treatment course, 95 versus 67% (P=0.01). The average duration of HCV treatment in cannabis users was 38 weeks compared with 33 weeks for the non-users.
Methods
Study setting and eligibility Recruitment and treatment took place at OASIS (Organization to Achieve Solutions in Substance-Abuse), a community-based non-profit clinic providing medical and psychiatric treatment to substance users in Oakland, CA. Although the clinic does not provide methadone treatment, comprehensive primary medical and psychiatric care services are provided on-site. All experimental procedures were followed in accordance with the Helsinki Declaration of 1975, as revised in 1983, and were approved by the Ethical Review Committee (Kansas City, Missouri, USA).
Men and women aged 18 years and older were considered eligible if they had been maintained on methadone for a period of 3 months or more and had a positive HCV polymerase chain reaction (PCR). Patients with non-HCV-related liver disease or decompensated liver disease were excluded. Those with untreated depression were excluded until stabilized on antidepressant treatment. Drug use was assessed by self-report as well as by random monthly urine toxicology testing, as per standard protocol at the methadone clinics.
Medications
HCV treatment consisted of IFN-a 2b, 3 x 106 units administered subcutaneously three times a week and ribavirin capsules, 1000 mg taken orally daily in two divided doses for patients weighing less than 165 lb, or 1200 mg daily for those weighing 165 lb or more. Patients were initially treated for 48 weeks regardless of genotype; however, subsequent data supporting the efficacy of 24 weeks of treatment for genotypes 2 and 3 led to a protocol amendment that shortened the treatment course for patients with these genotypes. Medications were selfadministered unless patients specifically requested otherwise.
Cannabis use
The use of cannabis during study was neither endorsed nor prohibited by study staff, and all patients obtained their cannabis outside the construct of the study protocol. However, because marijuana use was legalized for medical use in the state of California, it was often obtained with outside medical approval through local ‘cannabis clubs’. Cannabis use was quantified by self-report, with ‘regular’ use defined as the use of cannabis every day or every other day for a minimum duration of 4 weeks; ‘occasional’ reflected the use of less than daily quantities.
Procedures
After providing informed consent, participants completed a questionnaire that elicited baseline demographic, psychosocial, psychiatric, and substance use characteristics. The duration of HCV infection was estimated as one less than the number of years since injection drug use was initiated. Liver biopsy was suggested but not required, and was scored on the METAVIR scale of 0-4, with 0, none; 1, minimal-mild; 2, mild-moderate; 3, moderate-severe; and 4, cirrhosis.
Patients were monitored for treatment-related neutropenia, thrombocytopenia, and hemolytic anemia using standard published algorithms, and medication doses were adjusted accordingly [38]. Drug and alcohol consumption were assessed by monthly self-report questionnaires, and monthly random urine drug test results were obtained from the subject’s methadone treatment program. An HCV-RNA PCR was performed at baseline, at 6 months, at the end of treatment, and 6 months after the completion of therapy. Substance use during HCV therapy was actively discouraged, but did not result in treatment discontinuation unless the patient became unreliable in attending appointments or the clinician felt it represented a safety risk. HCV treatment was discontinued if requested by the patient, or for severe cytopenias, uncontrolled or worsening psychiatric conditions, or decompensating liver disease. The protocol was evaluated and approved by the Ethical Review Committee, Kansas City, Missouri, USA.
Outcome measures
The primary study endpoint was sustained virological response (SVR), as determined by undetectable levels of HCV RNA on analysis 6 months after the completion of therapy using the Bayer HCV-RNA branched DNA 3.0 assay, with a lower limit of detection of 550 IU/ml. Patients were classified as sustained virological responders at this time point if they had no detectable virus, or as non-responders if the PCR was positive. End-oftreatment response was defined as undetectable levels of HCV RNA at the completion of therapy. All analyses were performed on an intent-to-treat basis.
Adherence
Adherence to interferon was assessed by the timing of returned empty interferon vials and by a monthly questionnaire that detailed the number of missed doses of medication. Adherence to ribavirin therapy was assessed by pill counting and by query during a monthly questionnaire. Using adherence criteria developed by others, patients were considered adherent to the HCV treatment regimen if they took 80% or more of the prescribed interferon and 80% or more of the prescribed ribavirin for at least 80% of the projected treatment course [9].
Statistical analysis
All data were compiled in and analysed using SPSS version 11.5.0 for Windows (SPSS Inc., Chicago, Illinois, USA). Associations between outcome measures and cannabis use were determined using the Student’s t or Wilcoxon signed rank test for categorical variables. Bivariate analysis of categorical data was performed using the chi-square and Fisher’s exact tests. P values less than 0.05 in two-tailed comparisons were considered statistically significant. Logistic regression was used to assess for statistical independence among variables that showed a univariate association with a P value of 0.20 or less.