Smokin Moose
Fallen Cannabis Warrior & Ex Moderator
6.1 ~ The Cannabinoids
Cannabis' notorious resin is a complex mixture of cannabinoids, terpenes, and waxes, etc. There are about 100 known cannabinoids that occur only in hemp, with the exception of Cannabichromene, which is found in a few other plants. The entire hemp plant contains several hundred known chemicals.(1-3)
The cannabinoids are thought to be formed by condensation of monoterpene derivatives such as geraniol phosphate with a depside-type olivetolic acid. This leads initially to the formation of Cannabigerol (CBG) and Cannabichromene (CBC) and their carboxylic acids, then to Cannabidiolic Acid (CBDA), which undergoes ring closure to form TetraHydroCannabinol (THC) and its acid (THCA). The latter decarboxylates to form THC. Other biogenetic pathways featuring CBC have been proposed by De Faubert Maunder and by Turner and Hadley. (4, 5) (Fig. 6.1)
Figure 6.1 ~ Cannabinoids
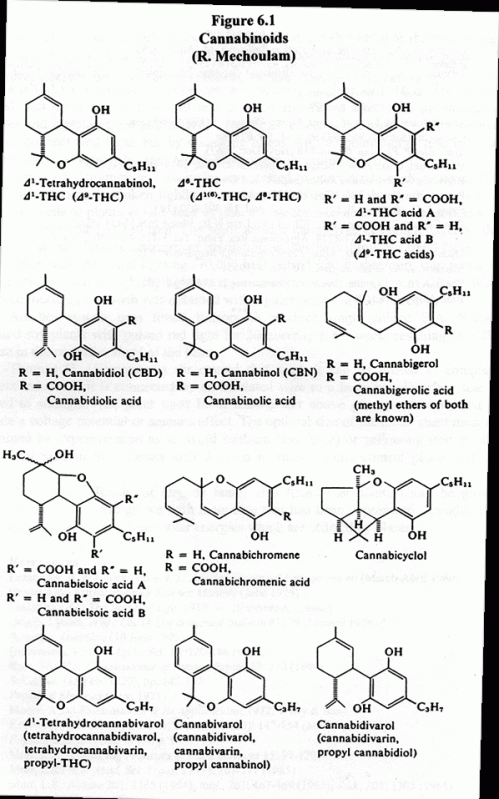
The acids comprise up to 40% of the cannabinoid content of young plants. THC dehydrogenates to form Cannabidiol (CBD). THC is a primary psychoactive cannabinoid. The minor constituent Cannabiverol (CBV) possesses only about 20% of THC's activity. CBD and CBN are not psychoactive, but they have valuable medical properties. (6-10)
Many synthetic analogs of THC are more or less potent than the parent molecule. The dimethylheptyl derivative is over 50 times more active, with effects lasting several days. Some nitrogen and sulfur analogs also are psychoactive.
The total synthesis of THC has been accomplished in many ways, most of which are difficult. However, the extraction of cannabinoids, their purification, isomerization and acetylation are easy experiments for dilettante souffleurs who would possess this elixir.
6.2 Extraction ~
Cannabis must be dried be it is extracted, because it is not possible to remove more than 50% of the cannabinoids from fresh material THC-Acid is difficult to extract If you plant to convert the THCA to THC, the plant material should be thoroughly decarboxylated by heating it under nitrogen at 105° C for 1 hour before performing a solvent extraction.
Chloroform is the most efficient solvent for the extraction of THC from cannabis. A single extraction will remove 98-99% of the cannabinoids within 30 minutes. A second extraction removes only 88-99% of the cannabinoids within 30 minutes. A second extraction removes 100% of the THC. Light petroleum ether (60-80°) also works well, but a single extraction removes only 88-95% of the cannabinoids; a double extraction removes up to 99%. Ethanol also can be used, but it removes ballast pigments and sugars which complicate the purification of the resin (11, 12)
Extract the dried cannabis with a suitable solvent for several hours at room temperature or by refluxing. Filter through charcoal to clarify the solution, then chill overnight to precipitate waxes, then filter the solution again. Concentrate it to one-half volume, and extract it with 2% aqueous sodium sulfate (to prevent oxidation). Separate the aqueous layer, and strip the solvent. The residue is crude hemp oil.
The odoriferous terpenes can be removed by steam or vacuum distillation. Cautious distillation in vacuo yields a fraction of crude red oil (bp 100-220° C/3 mm). This can be purified by redistillation or column chromatography. Use ethanol to remove the residue from the flask while it is still hot. Filter the solution through charcoal, and strip the solvent. Distill the residue to yield pure red oil (bp 175-195° C /2 mm). Distillation must be stopped if smoke appears, indicating decomposition. (13, 14)
Because THC is heat-sensitive, it is preferable to isolate the cannabinoids by column chromatography. The simplest method of column chromatography is performed with ethanol and ether extracts of hemp on alumina, yielding two major fractions: (1) chlorophyll, CBD, and CBN, and (2) THC. A second, more difficult method is performed on Florisil (use 10 times the weight of the oil) with the solvent system hexane:2% methanol. This yields a doubly-concentrated, viscous oil which can be repeatedly chromatographed on alumina to separate the THC and CBD. (15)
6.3 Isomerization ~
The potency of marijuana can be increased by about 50% simply by simmering a water slurry of the material for 2 hours. Add water as necessary to maintain the level. Cool and filter the mixture, and refrigerate the aqueous solution. Dry the leaf material at low heat. Drink the tea before smoking the marijuana. The effects are much more intense and last longer than those from the untreated leaves. The boiling water treatment isomerizes the inactive CBD, and decarboxylates THCA to THC.
Although Cannabidiol (CBD) has no psychoactivity, it does antagonize THC and produces other valuable sedative, antibiotic, and anti-epileptic effects. CBD can be isomerized to THC. If the plant is Phenotype III (containing mainly CBD in its resin), isomerization can double the yield of THC.
The CBD fraction of column chromatography can be distilled (bp 187-190° C/2 mm; pale yellow resin) to purify it. Isomerization can be accomplished with any of several solvents and acids. Alcohol and sulfuric acid isomerizes only 50-60% of CBD to THC; p-TolueneSulfonic Acid (p-TSA) in petroleum ether or other light, non-polar solvent will convert 90% of CBD to THC upon refluxing 1 hour at 130° F. (16, 17)
Reflux 3 gr CBD in 100 ml dry benzene for 2 hours with 200 mg p-TSA monohydrate until the alkaline Beam test (5% KOH in ethanol) is negative (no color). The Beam test gives a deep violet color with CBD. Separate the upper layer, wash it with 5% sodium bicarbonate, wash again with water, and strip the solvent. The remaining viscous oil should give a negative reaction to the Beam test. The crude THC can be purified by distillation (bp 169-172° C/0.03 mm), or by chromatography in 25 ml pentane on 300 gr alumina. Elute with pentane 95:5 ether to yield fraction of CBD and THC. Combine the THC fractions and distill (bp 175-178° C/1 mm).
Reflux 2 gr CBD in 35 ml cyclohexane, and slowly add a few drops of sulfuric acid. Continue to reflux until the Beam test is negative. Separate the sulfuric acid from the reaction mixture. Wash the solution twice with aqueous sodium bicarbonate, the twice again with water. Purify by chromatography, or distill (bp 165° C/0.01 mm). Any unreacted CBD can be recycled.
Another method is to reflux a mixture of 6 gr dry pyridine hydrochloride and 3 gr CBD at 125° C until the Beam test is negative. Wash the reaction mixture with water to remove the pyridine, then extract the mixture with ether. Wash the ether with water, evaporate the ether, and distill the residue i.v. to yield pure THC.
Similarly, reflux 3 gr CBD in 150 ml ethanol with 50 ml 85% phosphoric acid until the Beam test is negative. Work up the reaction mixture, and purify the THC.
Alternatively, reflux 3 gr CBD in 100 ml absolute ethanol containing 0.05% HCl for 19 hours. Extract the ether, wash the ether with water, dry, evaporate, and chromatograph on 400 gr alumina to yield:
(a) 0.5 gr 1-EthoxyHexaHydro-CBN (EHH-CBN: mp 86-87° C); elute with pentane 98:2 ether. Recrystalize from methanol and water.
(b) 2 gr THC; elute with pentane 95:5 ether. Repeated chromatography will separate the less polar forms.
(c) 0.5 gr EHH-CBN, eluted with pentane 93:7 ether. It can be isomerized to THC by refluxing in benzene for 2 hours. Cool the reaction mixture, wash it with water; separate, dry, and strip the solvent layer i.v. to yield THC.
CBD also can be isomerized by irradiation of a cyclohexane solution in a quartz vessel with a mercury lamp (235-265 nm) for 20 minutes. Workup of the reaction mixture yields 7-13% THC. (18-20)
6.4 ~ Acetylation
THC gives an acetate (ATHC) which is as potent as THC. The mental effects are quite subtle and pleasant. Wohlner, et al., prepared ATHC by refluxing the crude distillate of cannabis oil with approximately 3 volumes of acetic anhydride. It is purified by distillation i.v. or with steam.
Cahn prepared ATHC thus: add 150 ml acetyl chloride (dropwise with stirring and cooling) to 185 gr crude resin in 500 ml dry pyridine. Crystals may separate during the addition, or on standing a few hours at room temperature. Pour the mixture into dilute hydrochloric acid/ice. Separate the oil, then dissolve it in ether. Wash this solution with dilute acid, then with aqueous sodium carbonate, and again with water. Dry the solution with calcium chloride. Strip the solvent and distill the residue (240-270 C°/20 mm). The mixture of acetylated cannabinoids is separated by dissolving 2 gr in 100 ml benzene and chromatography over silica (150-200 mesh). Elute with 800 ml benzene. Combine the washings and the original effluent solutions, then strip the benzene i.v. to recover about 60% yield of light yellow oil. The material remaining on the column contains CBD and other cannabinoid acetates which can be recovered with ethanol and worked up.(21)
6.5 ~ Identification
Colorimetric tests are the simplest method of identifying cannabinoids. Hundreds more sophisticated analytical methods have been developed, as a review of Chemical Abstracts will reveal.
The Beam test is relatively specific. It gives a purple color with 5% ethanolic KOH, based on the oxidation of CBD, CBG, etc., and their acids to hydroxyquinones. However, THC does not react to the Beam test. Only two plants (Rosemary and Salvia) out of 129 common species tested give a weakly positive reaction. Among some 50 pure vegetable substances such as mono- and sesqui-terpenes, aromatics, etc., only juglone, embelin, and alkyl dioxyquinone develop a color reaction close to that of Cannabis. The reaction is not always dependable; it can be absent if the ethanol is hot. (22, 23)
A modification of the Beam test uses absolute ethanol saturated with gaseous hydrogen chloride. When added to an extract of suspect material, it gives a cherry red color which disappears if water is added. However, the test also gives more or less similar red color reactions with pinene, tobacco, julep, sage, rosemary, and lavender, etc..
The colorimetric test of Duquenois and Moustapha is not so specific as the Beam test, but it is very sensitive. The test reacts to CBN and CBD, but not to THC:
Vanillin (0.4 gr, acetaldehyde (0.06 gr) and 20 ml 95% ethanol is stored in a bottle. Extract the plant material with petroleum ether, then filter it and evaporate the solvent. Add exactly 2 ml of reagent and 2 ml concentrated hydrochloric acid. Stir the mixture; it turns sea-green, then slate gray, followed by indigo within 10 minutes. It turns violet within 30 minutes and becomes more intense.
The Duquenois-Negm hydrogen peroxide/sulfuric acid test is suitable for following the development of the resin and its potency. Macerate cannabis in chloroform or light petroleum ether for several hours. Evaporate 0.2 ml of the extract in a porcelain dish. Add 2 drops 30% hydrogen peroxide and 0.5 ml concentrated sulfuric acid. Rotate the dish gently, and observe the color of the liquid after 5 minutes. A pink color indicates CBD; blood-red color indicates a high concentration of THC. Violet or strong brown indicates THC. CBN produces a green color which quickly turns green-brown. (24)
The identification of cannabinoids has been made irrefutable by the modern development of gas chromatography, especially when combined with mass spectrometry.
Laboratories which do not possess these technologies can use diode-array and programmable variable-wavelength ultraviolet absorption detectors in conjunction with thin-layer chromatography (TLC) or high-performance liquid chromatography (HPLC), or a combination of both, and make comparisons with published data in conjunction with the specific absorption spectrum for the cannabinoids (200-300 nm). The combination of these techniques can overcome the problem of errors due to interference which often occur when single methods are used. (25)
6.6 ~ Neurology
In 1984, Miles Herkenham and his colleagues at NIMH mapped the brain receptors for THC, using radioactive analogs of THC developed by Pfizer Central Research. They found the most receptors in the hippocampus, where memory consolidation occurs. There we translate the external world into a cognitive and spatial "map". Receptors also exist in the cortex, where higher cognition is performed. Very few receptors are found in the limbic brainstem, where the automatic life-support systems are controlled. This may explain why it is so difficult to die from an overdose of cannabis. The presence of THC receptors in the nasal ganglia --- an area of the brain involved in the coordination of movement --- may enable the cannabinoids to relieve spasticity. Some receptors are located in the spinal cord, and may be the site of the analgesic activity of cannabis. A few receptors are found in the testes. These may account for the effects of THC on spermatogenesis and as an aphrodisiac.
S. Munro, et al., located a peripheral CX5 receptor for cannabinoids in the marginal zone of the spleen. The Anandamide/cannabinoid receptor site, a protein on the cell surface, activates G-proteins inside the cell and leads to a cascade of other biochemical reactions which generate euphoria. (26-31)
The brain produces Anandamide (Arachidonylethanolamide), which is the endogenous ligand of the cannabinoid receptor. It was first identified by William Devane and Raphael Mechoulam, et al., in 1992. Anandamide has biological and behavioral effects similar to THC. Devane named the substance after the Sanskrit word Ananda (Bliss). The discovery of Anandamide and its receptor site has unlocked the door to the world of cannabinoid pharmacology. (32-35)
CBD antagonizes THC and competes with THC to fill the cannabinoid receptor site. THC also exerts an inhibitory effect on acetylcholine activity through a GABA-ergic mechanism. It significantly increases the intersynaptic levels of serotonin by blocking its reuptake into the presynaptic neuron. THC also elevates the brain level of 5-hydroxy-tryptamine (5-HT) while antagonizing the peripheral actions of 5-HT. (36-39)
In 1990, Patricia Reggio, et al., developed a molecular reactivity template for the design of cannabinoid analgesics with minimal psychoactivity. The analgesic activity of the template molecule (9-nor-9b-OH-HHC) is attributed to the presence and positions of two regions of negative potential on top of the molecule. The template places all cannabinoid analgesics on a common map, no matter how dissimilar their structures. (40)
Cannabis' notorious resin is a complex mixture of cannabinoids, terpenes, and waxes, etc. There are about 100 known cannabinoids that occur only in hemp, with the exception of Cannabichromene, which is found in a few other plants. The entire hemp plant contains several hundred known chemicals.(1-3)
The cannabinoids are thought to be formed by condensation of monoterpene derivatives such as geraniol phosphate with a depside-type olivetolic acid. This leads initially to the formation of Cannabigerol (CBG) and Cannabichromene (CBC) and their carboxylic acids, then to Cannabidiolic Acid (CBDA), which undergoes ring closure to form TetraHydroCannabinol (THC) and its acid (THCA). The latter decarboxylates to form THC. Other biogenetic pathways featuring CBC have been proposed by De Faubert Maunder and by Turner and Hadley. (4, 5) (Fig. 6.1)
Figure 6.1 ~ Cannabinoids

The acids comprise up to 40% of the cannabinoid content of young plants. THC dehydrogenates to form Cannabidiol (CBD). THC is a primary psychoactive cannabinoid. The minor constituent Cannabiverol (CBV) possesses only about 20% of THC's activity. CBD and CBN are not psychoactive, but they have valuable medical properties. (6-10)
Many synthetic analogs of THC are more or less potent than the parent molecule. The dimethylheptyl derivative is over 50 times more active, with effects lasting several days. Some nitrogen and sulfur analogs also are psychoactive.
The total synthesis of THC has been accomplished in many ways, most of which are difficult. However, the extraction of cannabinoids, their purification, isomerization and acetylation are easy experiments for dilettante souffleurs who would possess this elixir.
6.2 Extraction ~
Cannabis must be dried be it is extracted, because it is not possible to remove more than 50% of the cannabinoids from fresh material THC-Acid is difficult to extract If you plant to convert the THCA to THC, the plant material should be thoroughly decarboxylated by heating it under nitrogen at 105° C for 1 hour before performing a solvent extraction.
Chloroform is the most efficient solvent for the extraction of THC from cannabis. A single extraction will remove 98-99% of the cannabinoids within 30 minutes. A second extraction removes only 88-99% of the cannabinoids within 30 minutes. A second extraction removes 100% of the THC. Light petroleum ether (60-80°) also works well, but a single extraction removes only 88-95% of the cannabinoids; a double extraction removes up to 99%. Ethanol also can be used, but it removes ballast pigments and sugars which complicate the purification of the resin (11, 12)
Extract the dried cannabis with a suitable solvent for several hours at room temperature or by refluxing. Filter through charcoal to clarify the solution, then chill overnight to precipitate waxes, then filter the solution again. Concentrate it to one-half volume, and extract it with 2% aqueous sodium sulfate (to prevent oxidation). Separate the aqueous layer, and strip the solvent. The residue is crude hemp oil.
The odoriferous terpenes can be removed by steam or vacuum distillation. Cautious distillation in vacuo yields a fraction of crude red oil (bp 100-220° C/3 mm). This can be purified by redistillation or column chromatography. Use ethanol to remove the residue from the flask while it is still hot. Filter the solution through charcoal, and strip the solvent. Distill the residue to yield pure red oil (bp 175-195° C /2 mm). Distillation must be stopped if smoke appears, indicating decomposition. (13, 14)
Because THC is heat-sensitive, it is preferable to isolate the cannabinoids by column chromatography. The simplest method of column chromatography is performed with ethanol and ether extracts of hemp on alumina, yielding two major fractions: (1) chlorophyll, CBD, and CBN, and (2) THC. A second, more difficult method is performed on Florisil (use 10 times the weight of the oil) with the solvent system hexane:2% methanol. This yields a doubly-concentrated, viscous oil which can be repeatedly chromatographed on alumina to separate the THC and CBD. (15)
6.3 Isomerization ~
The potency of marijuana can be increased by about 50% simply by simmering a water slurry of the material for 2 hours. Add water as necessary to maintain the level. Cool and filter the mixture, and refrigerate the aqueous solution. Dry the leaf material at low heat. Drink the tea before smoking the marijuana. The effects are much more intense and last longer than those from the untreated leaves. The boiling water treatment isomerizes the inactive CBD, and decarboxylates THCA to THC.
Although Cannabidiol (CBD) has no psychoactivity, it does antagonize THC and produces other valuable sedative, antibiotic, and anti-epileptic effects. CBD can be isomerized to THC. If the plant is Phenotype III (containing mainly CBD in its resin), isomerization can double the yield of THC.
The CBD fraction of column chromatography can be distilled (bp 187-190° C/2 mm; pale yellow resin) to purify it. Isomerization can be accomplished with any of several solvents and acids. Alcohol and sulfuric acid isomerizes only 50-60% of CBD to THC; p-TolueneSulfonic Acid (p-TSA) in petroleum ether or other light, non-polar solvent will convert 90% of CBD to THC upon refluxing 1 hour at 130° F. (16, 17)
Reflux 3 gr CBD in 100 ml dry benzene for 2 hours with 200 mg p-TSA monohydrate until the alkaline Beam test (5% KOH in ethanol) is negative (no color). The Beam test gives a deep violet color with CBD. Separate the upper layer, wash it with 5% sodium bicarbonate, wash again with water, and strip the solvent. The remaining viscous oil should give a negative reaction to the Beam test. The crude THC can be purified by distillation (bp 169-172° C/0.03 mm), or by chromatography in 25 ml pentane on 300 gr alumina. Elute with pentane 95:5 ether to yield fraction of CBD and THC. Combine the THC fractions and distill (bp 175-178° C/1 mm).
Reflux 2 gr CBD in 35 ml cyclohexane, and slowly add a few drops of sulfuric acid. Continue to reflux until the Beam test is negative. Separate the sulfuric acid from the reaction mixture. Wash the solution twice with aqueous sodium bicarbonate, the twice again with water. Purify by chromatography, or distill (bp 165° C/0.01 mm). Any unreacted CBD can be recycled.
Another method is to reflux a mixture of 6 gr dry pyridine hydrochloride and 3 gr CBD at 125° C until the Beam test is negative. Wash the reaction mixture with water to remove the pyridine, then extract the mixture with ether. Wash the ether with water, evaporate the ether, and distill the residue i.v. to yield pure THC.
Similarly, reflux 3 gr CBD in 150 ml ethanol with 50 ml 85% phosphoric acid until the Beam test is negative. Work up the reaction mixture, and purify the THC.
Alternatively, reflux 3 gr CBD in 100 ml absolute ethanol containing 0.05% HCl for 19 hours. Extract the ether, wash the ether with water, dry, evaporate, and chromatograph on 400 gr alumina to yield:
(a) 0.5 gr 1-EthoxyHexaHydro-CBN (EHH-CBN: mp 86-87° C); elute with pentane 98:2 ether. Recrystalize from methanol and water.
(b) 2 gr THC; elute with pentane 95:5 ether. Repeated chromatography will separate the less polar forms.
(c) 0.5 gr EHH-CBN, eluted with pentane 93:7 ether. It can be isomerized to THC by refluxing in benzene for 2 hours. Cool the reaction mixture, wash it with water; separate, dry, and strip the solvent layer i.v. to yield THC.
CBD also can be isomerized by irradiation of a cyclohexane solution in a quartz vessel with a mercury lamp (235-265 nm) for 20 minutes. Workup of the reaction mixture yields 7-13% THC. (18-20)
6.4 ~ Acetylation
THC gives an acetate (ATHC) which is as potent as THC. The mental effects are quite subtle and pleasant. Wohlner, et al., prepared ATHC by refluxing the crude distillate of cannabis oil with approximately 3 volumes of acetic anhydride. It is purified by distillation i.v. or with steam.
Cahn prepared ATHC thus: add 150 ml acetyl chloride (dropwise with stirring and cooling) to 185 gr crude resin in 500 ml dry pyridine. Crystals may separate during the addition, or on standing a few hours at room temperature. Pour the mixture into dilute hydrochloric acid/ice. Separate the oil, then dissolve it in ether. Wash this solution with dilute acid, then with aqueous sodium carbonate, and again with water. Dry the solution with calcium chloride. Strip the solvent and distill the residue (240-270 C°/20 mm). The mixture of acetylated cannabinoids is separated by dissolving 2 gr in 100 ml benzene and chromatography over silica (150-200 mesh). Elute with 800 ml benzene. Combine the washings and the original effluent solutions, then strip the benzene i.v. to recover about 60% yield of light yellow oil. The material remaining on the column contains CBD and other cannabinoid acetates which can be recovered with ethanol and worked up.(21)
6.5 ~ Identification
Colorimetric tests are the simplest method of identifying cannabinoids. Hundreds more sophisticated analytical methods have been developed, as a review of Chemical Abstracts will reveal.
The Beam test is relatively specific. It gives a purple color with 5% ethanolic KOH, based on the oxidation of CBD, CBG, etc., and their acids to hydroxyquinones. However, THC does not react to the Beam test. Only two plants (Rosemary and Salvia) out of 129 common species tested give a weakly positive reaction. Among some 50 pure vegetable substances such as mono- and sesqui-terpenes, aromatics, etc., only juglone, embelin, and alkyl dioxyquinone develop a color reaction close to that of Cannabis. The reaction is not always dependable; it can be absent if the ethanol is hot. (22, 23)
A modification of the Beam test uses absolute ethanol saturated with gaseous hydrogen chloride. When added to an extract of suspect material, it gives a cherry red color which disappears if water is added. However, the test also gives more or less similar red color reactions with pinene, tobacco, julep, sage, rosemary, and lavender, etc..
The colorimetric test of Duquenois and Moustapha is not so specific as the Beam test, but it is very sensitive. The test reacts to CBN and CBD, but not to THC:
Vanillin (0.4 gr, acetaldehyde (0.06 gr) and 20 ml 95% ethanol is stored in a bottle. Extract the plant material with petroleum ether, then filter it and evaporate the solvent. Add exactly 2 ml of reagent and 2 ml concentrated hydrochloric acid. Stir the mixture; it turns sea-green, then slate gray, followed by indigo within 10 minutes. It turns violet within 30 minutes and becomes more intense.
The Duquenois-Negm hydrogen peroxide/sulfuric acid test is suitable for following the development of the resin and its potency. Macerate cannabis in chloroform or light petroleum ether for several hours. Evaporate 0.2 ml of the extract in a porcelain dish. Add 2 drops 30% hydrogen peroxide and 0.5 ml concentrated sulfuric acid. Rotate the dish gently, and observe the color of the liquid after 5 minutes. A pink color indicates CBD; blood-red color indicates a high concentration of THC. Violet or strong brown indicates THC. CBN produces a green color which quickly turns green-brown. (24)
The identification of cannabinoids has been made irrefutable by the modern development of gas chromatography, especially when combined with mass spectrometry.
Laboratories which do not possess these technologies can use diode-array and programmable variable-wavelength ultraviolet absorption detectors in conjunction with thin-layer chromatography (TLC) or high-performance liquid chromatography (HPLC), or a combination of both, and make comparisons with published data in conjunction with the specific absorption spectrum for the cannabinoids (200-300 nm). The combination of these techniques can overcome the problem of errors due to interference which often occur when single methods are used. (25)
6.6 ~ Neurology
In 1984, Miles Herkenham and his colleagues at NIMH mapped the brain receptors for THC, using radioactive analogs of THC developed by Pfizer Central Research. They found the most receptors in the hippocampus, where memory consolidation occurs. There we translate the external world into a cognitive and spatial "map". Receptors also exist in the cortex, where higher cognition is performed. Very few receptors are found in the limbic brainstem, where the automatic life-support systems are controlled. This may explain why it is so difficult to die from an overdose of cannabis. The presence of THC receptors in the nasal ganglia --- an area of the brain involved in the coordination of movement --- may enable the cannabinoids to relieve spasticity. Some receptors are located in the spinal cord, and may be the site of the analgesic activity of cannabis. A few receptors are found in the testes. These may account for the effects of THC on spermatogenesis and as an aphrodisiac.
S. Munro, et al., located a peripheral CX5 receptor for cannabinoids in the marginal zone of the spleen. The Anandamide/cannabinoid receptor site, a protein on the cell surface, activates G-proteins inside the cell and leads to a cascade of other biochemical reactions which generate euphoria. (26-31)
The brain produces Anandamide (Arachidonylethanolamide), which is the endogenous ligand of the cannabinoid receptor. It was first identified by William Devane and Raphael Mechoulam, et al., in 1992. Anandamide has biological and behavioral effects similar to THC. Devane named the substance after the Sanskrit word Ananda (Bliss). The discovery of Anandamide and its receptor site has unlocked the door to the world of cannabinoid pharmacology. (32-35)
CBD antagonizes THC and competes with THC to fill the cannabinoid receptor site. THC also exerts an inhibitory effect on acetylcholine activity through a GABA-ergic mechanism. It significantly increases the intersynaptic levels of serotonin by blocking its reuptake into the presynaptic neuron. THC also elevates the brain level of 5-hydroxy-tryptamine (5-HT) while antagonizing the peripheral actions of 5-HT. (36-39)
In 1990, Patricia Reggio, et al., developed a molecular reactivity template for the design of cannabinoid analgesics with minimal psychoactivity. The analgesic activity of the template molecule (9-nor-9b-OH-HHC) is attributed to the presence and positions of two regions of negative potential on top of the molecule. The template places all cannabinoid analgesics on a common map, no matter how dissimilar their structures. (40)


